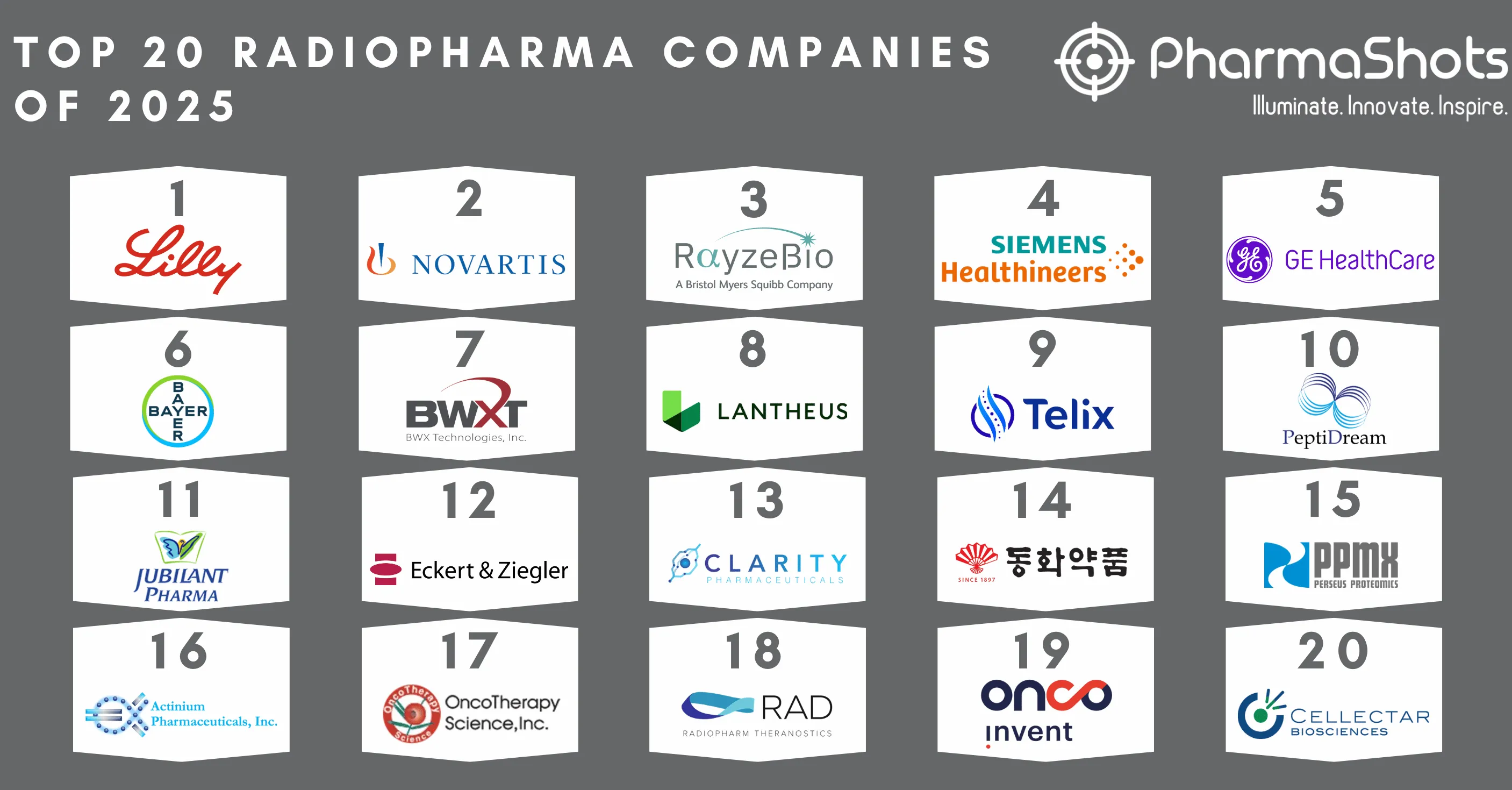
Actinium Entered into a License and Supply Agreement with Immedica for Iomab-B (131I apamistamab) in EU and MENA Countries
Shots:
- Actinium to receive $35M up front, $417M in regulatory & commercial milestones along with royalties
- Immedica obtains exclusive rights to commercialize Iomab-B in the EU & MENA countries while Actinium gets all rights related to Iomab-B in the US & ROW and will lead the clinical, regulatory activities & manufacturing of Iomab-B that is being developed for targeted conditioning to facilitate BMT & other cell & gene therapies
- Iomab-B is being evaluated in the P-III (SIERRA) trial in 150-patient aged ≥55yrs. with active, r/r AML at 24 transplant centers in the US and Canada while the results are expected in Q3’22. The therapy was licensed from the Fred Hutchinson Cancer Research Center
Ref: PR Newswire | Image: Actinium
Click here to read the full press release

Neha is a Senior Editor at PharmaShots. She is passionate and very enthusiastic about recent updates and developments in the life sciences and pharma industry. She covers Biopharma, MedTech, and Digital health segments along with different reports at PharmaShots. She can be contacted at connect@pharmashots.com.